Bihar Board 12th Chemistry Important Questions Long Answer Type Part 1 in English
Bihar Board 12th Chemistry Important Questions Long Answer Type Part 1 in English
Question 1.
What is u unit cell? How is it classified?
Answer:
Unit cell- Unit cells is the smallest portion of a crystal lattice which, when repeated in different directions generates the entire to lattice.
A unit cell is characterised by (i) its dimensions along the three edges, a, b and c. Those edges may or may not be mutually perpendicular.
(ii) Angle between the edges α (between b and c), β (Between a and c) and γ (between a and b).
Thus a unit cell is characterised by six parameters : a, b, c α, β and γ unit cells can be broadly divided into two categories-primitive and centred unit cells.
(i) Primitive unit cells- when constituent particles are present only on the comer position of a unit cells, it is called as primitive unit cell.
(ii) Centred unit cells- When a unit cells contains one or more constituent particles present at positions other that comers in addition to those at comers it is called a centred unit cell. Centred bit cells are of three types:
- Body centred unit cells- Such a unit cells contains one constituent particle (atom, molecules or ion) at its body-centre besides to ones that are at comers.
- Face centred unit cells- Such a unit cell containing one constituent particles present at the centre of its each face, besides the ones that are at its corners.
- End centred unit cells- In such a unit cell one constituent particle is present at the centre of any two opposite feces besides the one present at its comers.
In all there are seven types of primitive unit cells. They are cubic, tetragonal, orthorhombic, hexagonal, rhombohedral of trigonal monoclinic and triclinic.
Question 2.
What are the methods available to measure concentration of solution discuss them in brief?
Answer:
There are various ways to express the concentration of a solution
(1) Mass percentage- It may be defined as the number of parts by mass of a solute per 100 parts by mass of solution.
mass % of A =
where WA and WB are mass of solute and solvent respectively
(2) Volume percentage- Number of parts by volume of a solute per 100 parts by volume of solution.
Volume % of A =
Where VA and VB are volume of solute and solvent respectively.
(3) Normality (N)- 21 is defined as the numbers of gram equivalents of a solute dissolved per litre of the solution.
(4) Molarity (M)- It is defined as the number of grams moles of the solute dissolved per litre of the solution.
(5) Molarity (m)- It is defined as the number of gram moles of the solute dissolved per 100 gram of the solvent
(6) Mole fraction- It is defined as the ratio of the number of moles of a component to the total number of moles in the solution.
It for a binary solution NA and nB are the number of moles components A and B respectively then

(7) Parts per million (ppm)- It is the number of parts by mass of the solute per million parts by mass of the solution.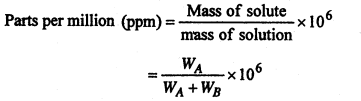
Where WA and WB are the masses of solute and solvent respectively.
Question 3.
Discuss the general Characteristics of group 15 elements.
Answer:
Nitrogen (IV), phosphorus (P), arsenic (As) antimony (Sb) anil bismuth (Bi) are members of group 15 elements.
(i) Electronic configuration- All the elements of group 15 have ns2np3 electronic configuration in their valence shells. The s-subshell is fully filled and p-subshell is exactly half filled (p1x, P1y, P1z). This inparss extra stability to their electronic configuration.
(ii) Oxidation state- The common oxidation states of these elements are -3, +3 and +5. The tendency to show -3 oxidation state decreases from top to bottom due to increase in atomic size and metallic character. N does not show +5 oxidation states because it has only 4 orbitals (on S three p) for bonding. Bi hardly forms any compound the -3 oxidation state. The stability of +5 oxidation decreases down the group and that of +3 state increases due to inert pair effect. N exhibits +1, +2 and +4 oxidation states when it reacts with oxygen. P also shows +1 and +4 oxidation states in some oxo-acids.
(iii) Atomic size- Atomic size increases from N to Bi due to addition of one orbit each time. This is a considerable increase in covalent radius from N to P. However from As to Bi only small increase in covalent radius is observed due to the presence of completely filled d and / or f orbitals in heavier elements.
(iv) Ionisation enthalpy- The ionisation enthalpy of group 15 elements much greater than that of group 14 elements due to the extra stability of half-filled p-orbitals and smaller size. It decreases from top to bottom due to gradual increase in atomic size.
(v) Electronegativity- Electronegativity decreases from top to bottom in the group. The difference of electronegativity is not much pronounced amongst the heavier elements.
Question 4.
[NiCl4]2- is paramagnetic while [Ni(Co4)] is diamagnetic Though both are tetrahedral. Explain.
Answer:
The valence shell electronic configuration of Ni2+ ion is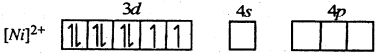
To accommodate four lone pair of electrons one 4s and three 4p orbitals hybridise to give four sp2 hybrid orbitals
Now four electron pairs donated by four Cl– ions inter into four sp3 hybrid orbitals and the confirmation of [NiCl4]2- ion becomes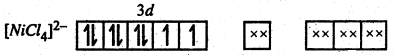
Since it contains two unpaired electrons, Hence its is paramagnetic.
Oxidation state of Ni in [Ni (CO4)] is zero. Hence it valence shell configuration is
CO is a strong field ligand; When four CO molecules approach to Ni atom the As electrons are forced to pair up with two unpaired electrons present in 3d sub shell. Thus are four vacant orbitals (one As and three 4p). In this case the valence shell configuration of Ni becomes.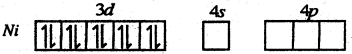
Now to accomodate four lone pairs of electrons donated by four CO molecules, the vacant four ortbitals hybridise to give four sp3 hybrid orbitals. The four low pairs of electrons enter into the four sp3 hybrid orbitals to from [Ni(CO)4] complex.
Since it does not contain any unpaired electron, hence it is diamagnetic.
Question 5.
(A) What do you understand by elevation of boiling point?
(B) What is meant by “Mobil elevation constant”? How is molal elevation constant related to the latent heat of vaporisation?
Answer:
Elevation of boiling point:
“Boiling point of a liquid is the temperature of which the vapour pressure of the liquid is equal to the atmospheric, pressure”. The addition of a non-volatile solute to the solvent lower its vapour pressure and thereby elevates the tailing point of the solution.
“The difference in the boiling point of the solution and the pure solvent is known as the elevation of boiling point of the solution”. It is denoted by “ΔTb” The elevation of boiling point of solution depends on nature of the solvent and the concentration of the solute.
If the boiling point of solution is T1°C and boiling point of solvent is T0°C
then Elevation of boiling point (ΔTb) = (ΔT1 – ΔT0)
(B) Molal elevation constant:
“The elevation in boiling point produced by dissolving 1 mole of a solute in 1000 gm of a solvent is called molal elevation constant”. It is denoted by “Kb”.
From Raoult’s first law we have-
Relation between molal elevation constant and latent heat of vaporisation-
Van’t Hoff co-related molal elevation constant (Kb) with the latent heat of vaporisation (Lν), thermodynamically that
Kb =
Where R = universal gas constant (cal or Joule).
Tb = boiling point of solvent in kelvin
Lν = latent heat of vaporisation per gram of solvent.
For water Tb = 373.15 K, R = 1.987 cal K-1 mol-1
and Lν = 540 cal g-1
∴ Kb =
Question 6.
State Kohlraush’s law.
Answer:
Kohlraush’s law :
Kohlraush in 1875 observed that die difference between the equivalent conductivity at infinite dilution of pairs of salts having an ion m common is constant at a constant temperature. For example the difference between at λ∞ 25°C of K and Na salts having same action is always 23.41 and that of bromide and chloride salts having same action is always 2.06.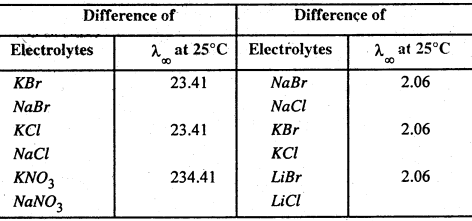
From these observations he concluded that every ion at infinite dilution contributed a definite amount towards the equivalent conductivity irrespective of the other ion with which it is associated in the electrolyte. Hence :
The equivalent conductivity at infinite dilution is for different electrolytes, the sum of two values on depending upon the cation and the other on the anion-
or, λ∞ =λa + λc (Kohlrausch’s law)
where the two values λ∞ and λc are called ionic mobilities or ionic conductances.
Question 7.
Present a comparative account of following :
(a) Shapes of HClO2 and HClO4
(b) Basic properties of PH3 and NH3
(c) Oxidising properties of F2 and Cl2
(d) Structure of O2 and sulphur.
Answer:
(a)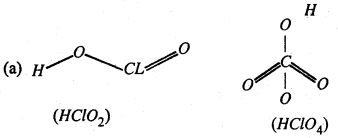
(b) NH3 is more basic than PH3 :
N is small in size whereas for P lone pair is scattered over whose of P. Therefore, it is difficult to donate the lone pair present on P in PH3.
(c) F2 is stronger oxidising agent than Cl2. The ready acceptance of an electron is the reason for the strong oxidising nature of halogens. Fluorine is more electronegative than chlorine. Hence fluorine is stronger oxidising agent then fluoring.
(d) Oxygen is a gas but sulphur is a solid. Due to small size and high electronegativity, oxygen form pπ-pπ multiple bonds and-exists as O2. They are held together by weak Van der Waals forces. Therefore oxygen is gas at room temperature.
Sulphur does not form pπ-pπ multiple bond because of bigger size. It forms S-S single bonds. The intermolecular forces of attraction between S8 are much stronger. Therefore, Sulphur is solid at room temperature.
Question 8.
An organic compound (A) having molecular formula C9H10O form an orange red precipitate (B) with 2, 4-DNP reagent compound (A) gives a yellow precipitate (C) when heated in the presence of iodine and NaOH along with a colourless compound (D). Compound (A) does not reduce Tollen’s reagent or Fehling’s solution nor does it decolourise bromine water. On drastic oxidation of (A) with chromic acid a carboxylic acid (E) of molecular formula C7H6O is formed. Deduce the structures of the organic compounds (A) to (E).
Answer: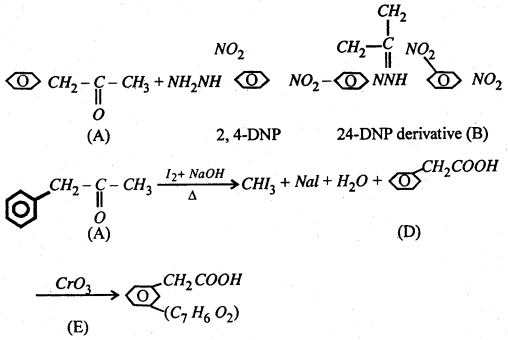
Reaction of (A) with 24-DNP indicates the presence of > C = 0 group.
(A) Does hot reduce Tollen’s reagent or Fehling’s solution. Therefore it must be a ketone.
(A) give iodoform test. Therefore, it is a methyl ketone i.e, having
(A) Does not decolourise Br2 water or Fehling’s reagent. This indicates the presence of unsaturation is due to aromatic ring.
Question 9.
What do you mean by abnormal molecular weight? Explain the season of this abnormality. Calculate the degree of dissociation of a substance with the help of abnormal molecular weight.
Answer:
Abnormal Molecular Weight- It is seen that the molecular weight of certain substances e.g., NaCl, KNO3 etc. in water when determined by any of the methods such as Osmotic pressure, lowering of vapour pressure, elevation in boiling point and depression in freezing point are less than their normal molecular weights. However molecular weight of certain substances like benzoic acid in benzene is greater than their normal molecular weight.
Reason of abnormality- The reason for this anomaly is that the substances either dissociate or associate in solutions. The molecular weight thus obtained are far from normal i.e., they are abnormal molecular weights. This fact is explained as follows-
We know that the osmotic pressure (P) is directly proportional to concentration (C), which in turn is proportional to the number of particles. Is also established that the Osmotic pressure is directly proportional to lowering of vapour pressure (ΔP) and the latter is proportional to elevation of boiling point and depression in freezing point (ΔT). All these properties are in turn inversely proportional to molecular weight of the substance.
Mathematically,
P ∝ C ∝ Number of particles ∝ ΔP ∝ ΔT ∝
i.e. Mol. wt. ∝
In dissociation, the number of particles increase and so the observed weight is less than the normal molecular weight. In association, the number of particles decreases and thus, the observed molecular weight is more than the normal molecular weight. Thus,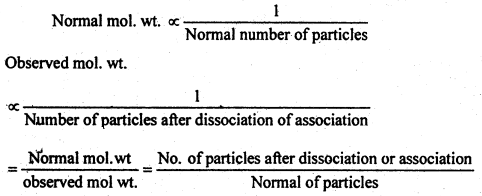
Calculation of degree of dissociation of the substance with the help of abnormal molecular weight-
The substance which dissociates or ionised in aqueous solution is called electrolyte. Their degree of dissociation is calculated as follow :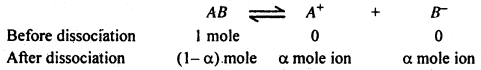
Let us suppose we1start with I mole of AB and am the degree of dissociation, mole ion of A+ and α mole of B+ is obtained after dissociation of α mole of AB.
Thus before dissociation no. of particles = 1
and after dissociation no. of particles = (1 – α + α + α) = (1 + α)
Hence,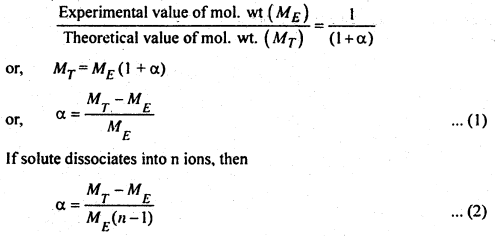
Question 10.
What do you mean by rate of reaction? What is its unit? What are the factors affecting the rate of reaction?
Answer:
Rate of reaction: It is defined as, the rate at which the concentration of a reactant changes with time. The rate at which a reaction proceeds can be followed by measuring the concentration at either a reactant or a product. If dx represents the amount of the reactant change during a smalHnterval of time dt, then the rate of reaction is represented by
Unit of rate-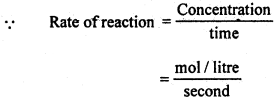
Factors affecting the rate of reaction-
Following are the factors which affect the rate of reaction-
- Concentration of reactants.
- Temperature.
- Nature of reactants.
- Catalyst
- Surface area of reactants.
All the above factors affect the rate of reaction.
