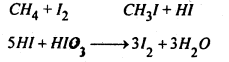Bihar Board 12th Chemistry Important Questions Long Answer Type Part 2 in English
Bihar Board 12th Chemistry Important Questions Long Answer Type Part 2 in English
Question 1.
(A) What do you mean by electrolysis? What are electrolytes and non-electrolytes? Give examples of each. What do you understand by weak and strong electrolytes? Give examples of each of them.
(B) State and explain Faraday’s law of electrolysis. How would you determine the equivalent of a element with its help?
(C) What do you mean by Electrochemical equivalent? Establish the relation between electrochemical equivalent and chemical equivalent of an element.
Answer:
(A) Electrolysis- The process of chemical decomposition of an electrolyte in their molten stage or in solution by passage of electric current is known as electrolysis.
The substances which allows the passage of electricity through its molten state or aqueous solution and causes electrical decomposition is called electrolyte.
ex.-bases and salts, are electrolyte.
The substance which behave like anon-conductor in molten stage or aquous solution is called non-electrolyte.
As- Urea, Benzene, Sugar, chloroform etc.
The electrolytes which ionise partially in their molten state aquous solution is called weak electrolyte. As-Acetic acid, Ammonium hydroride etc. The electrolytes which ionise completely in their molten state or aquous solution is called Strong Electrolyte.
As- HCl, NaCl, NaOH, HNO3, MgCl2 etc.
(B) Faraday proposed two laws regarding decomposition of electrolysis by an electric current. They are as follows-
(i) First Law of Electrolysis- This law states that the amount of a substance liberated at an electrode is directly proportional to the quantity of electricity passing through the electrolyte.
Thus, if . of substance deposited at electrode = W g.s.
Quantity of electricity = Q coulombs passed
Then, as per this law, w ∝ Q
or, W = ZQ
Where Z = (Electrochemical equivalent)
a constant of proportionality
As Q = c × t when camp, of current is passed for t time
W = Z × c × t Now, when c = 1 amp, t = 1 sec.
Then W = Z, So electrochemical equivalent is the amount of substance deposited at electrode when 4 amp/sec is passed through electrolyte.
(ii) Second Law of Electrolysis- It states that when same quantity of electricity is passed through different electrolytes, the amount of different substances deposited at the electrolytes is directly proportional to their equivalent Wt.
Let us consider same quantity of electricity is passed through three voltameters containing AgNO3, CuSO4 & H2SO4. Then as per this law we may write,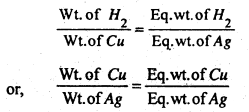
Determination of equivalent weight of element By Faraday’s Law- If A w1, w2, w3 etc. be the amount of substance deposited at different electrodes and E1, E2 … etc. be their respective equivalent weight. Then from Faraday’s 2nd law of electrolysis we may write,
W1 ∝ E1 ; W2 ∝ E2
or,
If amount of substance deposited at electrodes are known and equivalent wt. of one is known then other can be known by using the relation.
(C) EIcctrochemical equivalent- From 1st law of electrolysis of Faraday; We know that, amount of substance liberated at electrode during electrolysis is directly proportional to the quantity of electricity passing through the electrolyte.
∴ W ∝ c × t where, W = Wt. of substance deposited at electrode
c = Current passed for time t.
or, W = e × c × t;
e is constant of proportionality, called electrochemical equivalent.
if c = 1 amp, t = 1 second, then, W = e
i.e. Electrochemical equivalent is the amount of substance deposited b> one ampere of current passing for one second.
Electrochemical equivalent of any element =
Establishment of relation between electrochemical equivalent and chemical equivalent of element-It amount of substance deposited at different electrodes are W1, W2 … etc. and E1, E2 ….. etc. be their respective equivalent wt., then,
W1 ∝ E1; W2 ∝ E2
From 1st law of electrolysis
W1 = Z1 ct and W2 = Z2ct …….. (ii)
Where Z1 and Z2are electrochemical equivalents of substances.
From (i) and (ii) we may write-
or, Z ∝ E
i.e. electrochemical equivalent of any element is directly proportional to chemical equivalent of element.
It has been found experimentally that value of constant = 96500 coulomb = 1 Faraday.
∴ Electro chemical equivalent =
Question 2.
Discuss Physico-chemical principle of Haber’s process of manufacturing Ammonia.
Answer:
Physico-Chemical principle of manufacturing ammonia by Haber’s process. In Haber process, one volume nitrogen combines with three volumes of Hydrogen to form ammonia gas on a large scale.![]()
Above reaction is reversible and exothermic further as a result of reaction, there is a decrease in volume. Therefore, as per Le-Chatiliers principle, more ammonia would be formed at low temperature and high pressure.
But mach lowering of temperature leads to slow-down of the role of reaction. Thus reaction is generally, carried out at optimum temperature of 450°C – 500°C in presence of catalyst at 200 atmospheric pressure. Iron powder is generally used as catalyst. To activate catalyst MaO or Al2O3 and K2O mixture is used as promotor.
Further to facilitate more formation of ammonia, ammonia formed is quickly removed and condensed to prevent it from its decomposition to N2 and H2 as the reaction is reversible.
Question 3.
Describe the principal of preparation of SO2 gas in the laboratory. Discuss the following properties of SO2 – (a) Acidic properties (b) Bleaching properties (c) Oxidising properties and (d) Reducing properties.
Answer:
Preparation of SO2 gas in the laboratory-
Principle- SO2 gas is prepared in the laboratory by the action of Cu and conc. H2SO4
Cu + 2H2SO4 → CuSO4 + 2H2O + CO2
SO2 gas is collected in the gas jar by the displacement of air.
Following reaction of SO2 gas-
(a) Acidic properties- It is an acidic oxide. It reacts with water and forms sulphurous acid.
SO2 + H2O → H2SO3
(b) Bleaching Properties- It bleaches the colour of moist substances. Its bleaching properties is due to reduction. It combines with water and form sulphuric acid and nascent hydrogen which bleaches the coloured substance into colourless:
SO2 + 2H2O → H2SO4 + 2[H]
Colour + 2[H] → Colourless
(c) Oxidising Properties- It acts as an oxidising agent is some reactions.e.g.-
(i) It oxidises H2S into sulphur.
SO2 + 2H2S → 3S +2H2O
(ii) It oxidises iron powder into their oxide and sulphide.
SO2 + 3Fe → 2Fe0 + FeS
(iii) It oxidises SnCl2 in SnCl4
SO2 + 2SnCl2 + 4HCl → S + 2SnCl4 + 2H2O
(d) Reducing properties- Aqueous solution of SO2 is a strong reducing agent. When SO2 reacts with water produce nacesent hydrogen, which acts as strong reducing agent.
2H2O + SO2 → H2SO4 + 2[H]
(i) It reduces halogens (Cl2, Br2 and l2) in their halogen acids, e.g.
SO2 + Cl2 + 2H2O → 2HCl + H2SO4
SO2 + Br2 + 2H2O4 → 2HBr + H2SO4
SO2 + I2 + 2H2O → 2HI + H2SO4
(ii) It reduces ferric sulphate into ferrous sulphate.
Fe2(SO4)3 + SO2 + 2H2O → 2FeSO4 + 2H2SO4
(iii) It reduces acidic solution of potassium permanganate into manganese sulphate.
2KMnO4 + 5SO2 + 2H2O → 2MnSO4 + K2SO4 + 2H2SO4
Question 4.
What are transition metals? Discuss briefly the characteristic properties of transition elements.
Answer:
Transition Elements: Elements’having incompletely filled inner ‘d’ orbitals are called transition elements. The general electronic configuration is (n – 1) dx ns2 where x ranges, from 1 to 9. There are three series as first, second and third series of transition elements. In the first transition series 3d-orbitals are filled up in the second transition series 4d-orbitals and in the third transition series 5d-orbitals are filled up. Elements of group IIIB, IVB, VIB, VIIB, VIIIB and IB, IIB are classified as transition elements.
Characteristic Properties of Transition Elements-
1. All the transition elements are metals.
2. All these elements contain incompletely filed inner p-orbital.
3. Oxidation states- Transition elements have variable oxidation states and show variable valency. It is due to the involvement of electrons in the inner d-orbital.
Fe++(ous) → 3d5 Cr++ → 3d4 Mn++ → 3d5
Fe+12(ic) → 3d5 Cr+3(ic) → 3d3 Mn+3 → 3d4
Lower oxidation states are generally more stable for 1st transitions (-3d series)
4. Melting point- Transition metals have high melting points.
5. Atomic and Ionic radii- The outermost electronic configuration in (n – 1) d4 ns2 the newcomer electron is added to the (n – 1) dx orbital while ns2 and np0 orbitals remain unchanged. Thus due to d-block construction atomic and ionic radii in a series show very little variation.
6. Ionisation Potential- As variation in the atomic radii is very small, the variation in ionisation potential is also small. The ionisation potentials are practically constant in a transition metal series.
7. Colour salts (compounds)- Transition metals form salts e.g.
Fe++ salts → green
Fe+8 salts → yellow
Mn+2 salt → pink
Co+2 salts → pink.
The colour is due to either d-d transition or charge transfer.
8. Catalytic properties- Transition metals are good catalic agents e.g finely divided nickel, platinised asbestos, paladium, iron etc. are used as catalysts.
9. Magnetic properties- Transition metals, free ions and salts of lower oxidation states are paramagnetic due to the presence of unpaired electrons.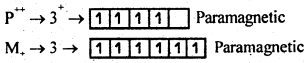
Fe, Co and Ni are very strongly paramagnetic and are called as Ferromagnetic.
Para-magnetism is expressed as :
μ =
Where μ = Magnetic moment. (Magnetic dipole moment)
n = number of unpaired electrons.
The value of magnetic moment is expressed by the unit Bohr magnetion (B.M.)
Example-![]()
Cr++ has 4d unpaired electrons, hence its n will be =
(VIII) has 3d0 hence its μ value is zero, Mn (VIII) is diamagnetic.
Now it can be shown from the quantum theory of atoms and ions that the magnetic moment due to unpaired electrons in the atom or in the atom or iron is given by
μ = 2
| Number of unpaired electrons | S | µ(B.M) |
| 1 | 1.73 | |
| 2 | 1 | 2.83 |
| 3 | 3.87 | |
| 4 | 2 | 4.90 |
| 5 | 5.92 |
10. Hydrolysis salts- Salts of transition metals are readily hydrolysed in aq. solution.
e.g. FeCl3 + 3H2O → Fe(OH)3 + 3HCl
Aq. solution of salts of transition metals are acidic. Transition metal cations are associated with some convinient characteristics due to the presence of incompletely filled ‘d’ orbitals.
Due to high charge density, the cation of a transition metal pulls ions pair of electron from oxygen atom in H2O molecule and a hydrate finally gets hydrolysed.
e.g. Fe+3 + H2O → (H2O:Fe+2) → Fe(OH)+2 + H+
11. Complex formation- Transition metals have a great tendency to form complex compounds (coordination compounds). This tendency is due to the presence, of incompletely filled ‘d’ orbitals and high effective nuclear charge (transition metals have low atomic volume). Transition metals and their cations can readily accept lone pair of electrons from ligands like NH3, CN–, H2O etc.
e.g.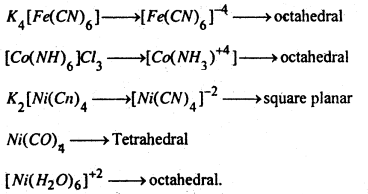
Question 5.
Describe the preparation, properties and structure of the following.
(1) Orthophosphoric acid (CH3PO4) (2) Metaphospharic acid (HPO3) and (3) Pyrophospharic acid (H4P2O7).
Answer:
(1) Orthophosphoric acid (H3PO4) –
Preparation-It is commonly known as phosphoric acid and is prepared by the following methods-
(i) By the hydrolysis of phosphorus pentachloride with excess of water.
PCl5 + 4H2O → H3PO4 + 5HCl
(ii) By dissolving phosphorus pentaoxide in hot water.
P2O5 + 3H2O → 2H3PO4
(iii) On commercial scale, it is prepared by the action of dilute H2SO4 on phosphoric rocks or bone ash.
Ca3(PO4)2 + 3H2SO4 → 3CaSO4 + 2H3PO4
(iv) Laboratory Method : It is prepared by heating phosphorus with conc. HNO3
P + 5HNO3 → H3PO4 + H2O + 5NO2
Properties-
(i) Orthophosphoric acid is a colourless, syrupy liquid.
(ii) Orthophosphoric acid is tribasic acid and forms three series of salt e.g., Sodium dihydrogen phosphate, (NaH2PO4) (primary phosphate); disodium hydrogen phosphate (NaHPO4) (Secondary phosphate) and normal Sodium phosphate Na3PO4 (tertiary phosphate).
(iii) It is a weak acid and undergoes ionization in three steps-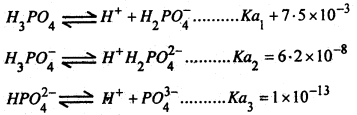
A solution of Na2HPO4 is practically neutral to litmus, that of NaH2PO4 is acidic while that of Na3PO4 alkaline to litmus.
(iii) It undergoes dehydration on heating.
(a) At 525K-Pyrophosphoric acid is obtained.
(b) At 875K-it forms metaphosphoric acid.
Structure-Since the acid is tribasic, die structure of phosphoric acid is understood to be as follows-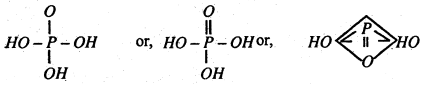
(2) Metaphosphoric acid (HPO3)
Preparation-(i) An equeous solution of die acid can be prepared by dissolving phosphorus pentoxide in cold water.
P4O10 + 2H2O → 4HPO3
(ii) It can be prepared by heating orthophosphoric acid or pyrophosphoric acid.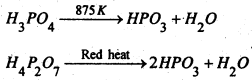
Properties-(i) Metaphosphoric acid is glassy deliquescent solid.
(ii) It is a monobasic and its salts are known as metaphosphates.
(iii) The equeous solution of the acid on boiling changes into orthophosphoric acid.
Structure- Since it is monobasic acid, it contains one-OH group linked to the central phosphorus atom. The acid is represented by the following structure.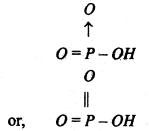
(3) Pyrophosphoric acid (H4P2O7) _
Preparation- This is obtained by heating orthophosphoric acid to 525K.
Properties- (i) Acidic nature-It is a tetrabasic acid but only two series of salts are commonly known the normal salts e.g., Na4P2O7 and diacid salts, eg., Na2H2P2O7
(ii) Action with water-When boiled with water, it changes into orthophosphoric acid.
(iii) Action of heat-On heating it to red-heat, it yields metaphosphoric acid.
Structure- Pyrophosphoric acid being tetra basic is given the following structure-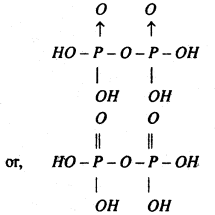
Question 6.
(A) What me interhalogen compound? Discuss different types of interhalogen compounds.
(B) Why are interhalogen more reactive than halogen?
Answer:
(A) The binary compounds formed by halogen among themselves are known, as interhalogen compounds. The halogen form various compound which are binary combination of halogens among themselves. With the V exception of BrCl, ICl, ICl3 and IBr the compound are all halogen fluorides. It will be noted that all the interhalogen compounds are of the type AXn where n is an odd number and X is always the lighter and smaller halogen than A. The value of n is always odd. This is odd number of unpaired electrons available in the ground state first, second and third excited states at the halogen atoms forming the interhalogen compounds.
These compounds can tie divided into following four types-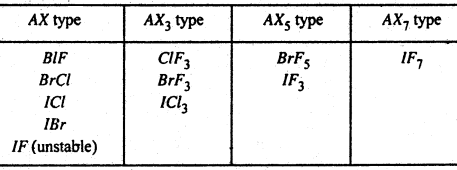
[Here A represents the larger halogen atom and X the smaller one]
The bonds in these compounds are essentially covalent because at the small electronegativity difference. The compounds formed ¡n the AX and AX3 group are those where the electronegativity difference is not too great the higher types AX5 and AX7 are shown by large atoms such as Br and I combining with small atoms such as F, because it is possible to pack more small atoms round a larger one.
(B) Interhalogen compound say at the type ClF, BrF and ClF3 have polarity in the bonds. Where as halogens say F2 or Cl2 have non-polar bands. A polar bond is easier to break compared to a non-polar bond. That is the reason why interhalogen compounds are more reactive the bonds in such compounds can be easily broken.
Question 7.
What are haloalkanes? How are they classified? How can haloalkanes be prepared from (1) alcohols (2) alkenes and (3) alkanes?
Answer:
Haloalkanes- The halogen derivatives of alkanes are called haloalkanes. These are classified as mono, di, tri- tetra haloalkanes etc. according as they contain one, two, three, four etc. halogen atoms respectively in their molecules, eg.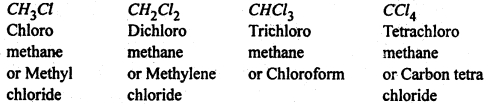
Classification of alkyl halides- Alkyl halides are classified as primary (1°), secondary (2°) and tertiary (3°) according to as halogen atom is attached to primary, secondary and tertiary carbon atoms respectively, eg.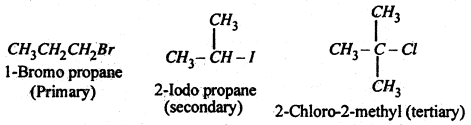
Methods of preparation of haloalkanes-
(1) From alcohols- The most convenient method for the preparation of haloalkanes involves the substitution of the -OH group of an alcohol by the. halogen atom. This can be done by the following methods:
(a) By the action of halogen acids- Alcohols are converted into haloalkanes by the action of halogen acids.![]()
For a given alcohol, the reactivity of the halogen acids in the above reaction are as follow : HI > HBr > HCl and that of alcohols for a given halogen acid follows die order: tertiary > secondary > primary i.e. 3° > 2° > 1°.
(i) Primary and secondary chloroalkane are prepared by passing HCl gas through a suitable alcohol in presence of anhydrous ZnCl2.
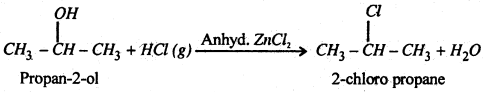
(ii) Tertiary alcohol, however, react readily with cone. HCl even in the absence of ZnCl2
Bromoalkanes or alkyl bromide are obtained by refluxing a suitable alcohol with constant boiling hydrobromic acid (48%) in presence of little cone. H2SO4 as catalyst.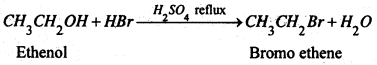
Iodoalkane or alkyl iodide are prepared by refluxing a suitable alcohol with constant boiling hydroiodic acid (57%).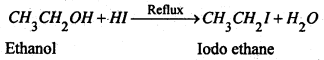
(b) By the action phosphorous halides-Ph0spherous halides react with alcohols to form haloalkane.
Chioroalkanes are repared by the action of either PCl5 or PCl3 on suitable alcohols.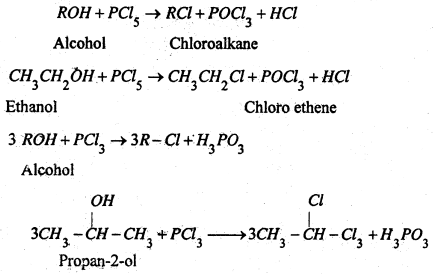
Similarly, Bromoalkane or iodoalkanes are prepared by the action of PBr3 and PI3 respectively on suitable alcohols.
P4 + 6Br2 → 4PBr3
P4 + 6I2 → 4PI3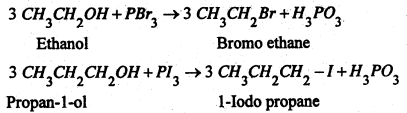
(C) By the action of thionly chloride- Chloroalkane are prepared by refluxing alcohols with thonyl chloride in presence of pyridine.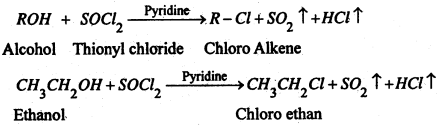
(2) From alkenes- Alkenes react with halogen acids to form haloalkanes. The order of reactivity of halogen acids are: HI > HBr > HCl.
The addition of HX to an unsymmetrical alkene takes place according to Markovnikov’s rale. e.g.
However, in presence of peroxides such as benzoyl peroxide, the addition of HBr (But not HCl or HI) to unsymmetrical alkenes takes place contain to Markovnikov’s rale. This is known as Peroxide effect or Kherasch effect, e.g.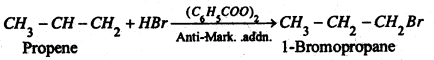
(3) From alkanes- Halogenation of alkanes With Cl2 or Br2 in presence of heaf or light usually gives a complex mixture of mono-, di- and polyhaloalkanes. E.g. chlorination of methane gives four products.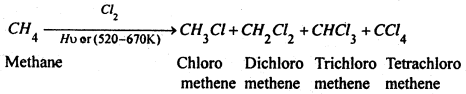
In case of higher alkanes even monohalogenation gives a mixture of all possible isomeric haloalkenes indiceting thereby that all types hydrogens whether 1 °, 2° or 3° are substituted by the halogen atom. e.g.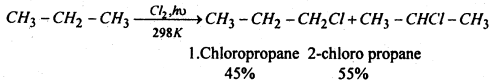
Iodination in reversible, but it may be carried out in the presence of an oxidising agent, such as HIO3, HNO3, AgO etc. which destroys the HI as it in formed.